Volume 17, Issue 4 (Jul-Aug 2023)
mljgoums 2023, 17(4): 1-4 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Matena M, N. Bongo G, Ngbanda H, B. Bakemo E, M. Mukaba Y, K. Munzumba J, et al . Biological evaluation of coagulation problems in COVID-19 patients hospitalized at the centre hospitalier mère-enfant monkole, kinshasa, Democratic Republic of the Congo. mljgoums 2023; 17 (4) :1-4
URL: http://mlj.goums.ac.ir/article-1-1613-en.html
URL: http://mlj.goums.ac.ir/article-1-1613-en.html
Merlin Matena1 

 , Gédéon N. Bongo2
, Gédéon N. Bongo2 

 , Honoré Ngbanda3
, Honoré Ngbanda3 
 , Eddy B. Bakemo4
, Eddy B. Bakemo4 
 , Yves M. Mukaba1
, Yves M. Mukaba1 
 , Jossard K. Munzumba1
, Jossard K. Munzumba1 
 , Justin M. Vuvu5
, Justin M. Vuvu5 
 , Christel K. Kande1
, Christel K. Kande1 
 , Jacques N. Ngayuna5
, Jacques N. Ngayuna5 
 , Jonathan I. Kukila6
, Jonathan I. Kukila6 
 , Ngbolua Koto-te-Nyiwa7
, Ngbolua Koto-te-Nyiwa7 



 , Gédéon N. Bongo2
, Gédéon N. Bongo2 

 , Honoré Ngbanda3
, Honoré Ngbanda3 
 , Eddy B. Bakemo4
, Eddy B. Bakemo4 
 , Yves M. Mukaba1
, Yves M. Mukaba1 
 , Jossard K. Munzumba1
, Jossard K. Munzumba1 
 , Justin M. Vuvu5
, Justin M. Vuvu5 
 , Christel K. Kande1
, Christel K. Kande1 
 , Jacques N. Ngayuna5
, Jacques N. Ngayuna5 
 , Jonathan I. Kukila6
, Jonathan I. Kukila6 
 , Ngbolua Koto-te-Nyiwa7
, Ngbolua Koto-te-Nyiwa7 

1- Service of Laboratory, Department of Medical Biology, Centre Hospitalier Mère et Enfant de Monkole, Kinshasa, Democratic Republic of the Congo/ Department of Medical Biology, Faculty of Health Sciences, Université PanAfricaine du Congo, Kinshasa, Democratic Republic of the Congo
2- Department of Life Sciences, Faculty of Science and Technology, University of Kinshasa, Democratic Republic of the Congo ,gedeonbongo@gmail.com
3- Department of Medical Biology, Faculty of Health Sciences, Université PanAfricaine du Congo, Kinshasa, Democratic Republic of the Congo
4- Unit of Biochemistry, Department of Medical Biology, Cliniques Universitaires de Kinshasa, Kinshasa, Democratic Republic of the Congo
5- Service of Laboratory, Department of Medical Biology, Centre Hospitalier Mère et Enfant de Monkole, Kinshasa, Democratic Republic of the Congo
6- Service of Laboratory, Department of Medical Biology, Centre Hospitalier Mère et Enfant de Monkole, Kinshasa, Democratic Republic of the Congo/ Unit of Biochemistry, Department of Medical Biology, Cliniques Universitaires de Kinshasa, Kinshasa, Democratic Republic of the Congo
7- Department of Life Sciences, Faculty of Science and Technology, University of Kinshasa, Democratic Republic of the Congo
2- Department of Life Sciences, Faculty of Science and Technology, University of Kinshasa, Democratic Republic of the Congo ,
3- Department of Medical Biology, Faculty of Health Sciences, Université PanAfricaine du Congo, Kinshasa, Democratic Republic of the Congo
4- Unit of Biochemistry, Department of Medical Biology, Cliniques Universitaires de Kinshasa, Kinshasa, Democratic Republic of the Congo
5- Service of Laboratory, Department of Medical Biology, Centre Hospitalier Mère et Enfant de Monkole, Kinshasa, Democratic Republic of the Congo
6- Service of Laboratory, Department of Medical Biology, Centre Hospitalier Mère et Enfant de Monkole, Kinshasa, Democratic Republic of the Congo/ Unit of Biochemistry, Department of Medical Biology, Cliniques Universitaires de Kinshasa, Kinshasa, Democratic Republic of the Congo
7- Department of Life Sciences, Faculty of Science and Technology, University of Kinshasa, Democratic Republic of the Congo
Full-Text [PDF 463 kb]
(893 Downloads)
| Abstract (HTML) (3184 Views)
Full-Text: (718 Views)
Introduction
Coronavirus 2019 (COVID-19) is a viral infection caused by the severe acute respiratory syndrome virus (SARS-CoV-2) (1, 2). SARS-CoV-2 is a single-stranded RNA virus that enters the body's cells via the angiotensin-converting enzyme 2 (ACE2) receptor (3, 4). This receptor is widely expressed in the body, particularly in the pulmonary alveoli and vascular endothelium (5, 6). WHO statistics on the coronavirus pandemic have revealed over 317 million infected cases and over 5 million deaths worldwide. Like all countries, the Democratic Republic of the Congo (DRC) was also affected by the COVID-19 pandemic, with 81,719 infected cases and 1225 deaths (7).
The disease is transmitted through close contact with infected persons. Most infected individuals initially present with respiratory failure, but some progress to more systemic disease and multi-organ dysfunction. The elderly and those with co-morbidities are at an increased risk of death from COVID-19 (8). The clinical manifestations reported during COVID-19 infection are vast. Among them, an inflammatory state can be very significant and lead to a cytokine storm and a prothrombotic state, resulting in thrombosis (9). This inflammation causes damage to the activation of the microvascular endothelium, which is probably at the origin of the manifestations of pulmonary or renal pathologies (9). Notably, 5% to 30% of hospitalized patients develop a clinically proven thrombotic event. Emerging evidence suggests that endothelial damage resulting from cell invasion by SARS-CoV-2 and subsequent dysregulation of the host response involving the inflammation and coagulation pathways play a crucial role in the progression of severe COVID-19 (10,11).
The most severely ill patients frequently observe coagulopathy and massive intravascular clot formation of the disseminated intravascular coagulation type. Therefore, coagulation and fibrinolysis tests help identify and monitor severe cases of COVID-19. Increased D-dimer levels, a relatively modest decrease in platelet count, and prolonged prothrombin time are typical findings in patients with COVID-19 and coagulopathy (12). This hemostatic imbalance is thought to contribute to organ failure, multisystem involvement, and death (13). In France, in a series of 107 consecutive COVID-19 cases admitted to an intensive care unit, 20% developed venous thromboembolism, despite standard pharmacological thromboprophylaxis and full-dose anticoagulation therapy. COVID-19 patients with acute respiratory syndrome were 6 times more likely to develop pulmonary embolism (13). However, the optimal way to prevent and treat the coagulopathies that accompany COVID-19 would be to identify early markers of increased risk of thrombosis and the thrombotic complications that may ensue to help physicians prevent such complications (12, 13). The pathophysiology of microthrombosis is not yet well understood, and several hypotheses have been put forward. In the DRC, nearly 15% of COVID-19 patients are followed in different COVID-19 Treatment Centers. Of all these hospitalized patients, 41.4% were on oxygen in a severe and critical condition, all in Kinshasa, with a case fatality rate of 10% (14).
This study aimed to evaluate the coagulation disorders in COVID-19 patients admitted to Centre Hospitalier Mère et Enfant Monkole (CHME-Monkole), Kinshasa, DRC, from a biological perspective.
Methods
This descriptive cross-sectional hospital-based study on patient files was conducted between July 2020 and June 2021 at the Medical Biology Laboratory of CHME-Monkole in Mont-Ngafula municipality, Kinshasa, DRC.
As inclusion criteria, any person infected with the SARS-CoV-2 virus who was admitted to the hospital for therapeutic follow-up at CHME-Monkole and had samples sent to the Medical Biology Laboratory for analysis was included in the study. On the contrary, any person infected with SARS-CoV-2 who was not admitted and followed up at CHME was excluded from this study. The study population comprised patients with COVID-19 admitted to this health facility, and the sample size was 130 patients determined using a random sampling technique after conducting interviews based on a questionnaire.
For each respondent, epidemiological, clinical, biochemical, and hematological data and socio-demographic characteristics were collected using a questionnaire. The determination of Prothrombin time (PT), the Activated Partial Thromboplastin time (APTT), platelet count, and D-dimers were performed following the protocols of Mohammed et al. (15), Yorike et al. (16), Bashash et al. (17), Elkhalifa (18), and Long et al. (19) with slight modifications. The normal ranges for PT were 10-15 seconds converted to a percentage (70-100%), for APTT were 30-40 seconds, for platelet count was 150,000-400,000/μL of blood, and for D-dimer was less than 500 μg/L (FEU). These values were used to confirm abnormal cases and to find correlations between these biological parameters (PT, APTT, D-dimers, and platelet count) and socio-demographic characteristics.
Data entry was performed in Microsoft Excel and then exported to SPSS 25.0 for coding and analysis. A descriptive analysis (frequency and percentage) was conducted for socio-demographic characteristics, while Pearson correlation (Chi-square) was used to determine associations between socio-demographic characteristics and different biological parameters. Conversely, the correlation was applied to determine the relationship between socio-demographic characteristics and D-dimer levels. The significance level for statistical tests was p < 0.05, and the confidence interval was 95%.
For data collection in this study, informed consent was sought from patients after explaining the purpose of the research. The confidentiality and anonymity of the patients' data were assured. The study was authorized by the Ethical Committee of CHME-Monkole (Ethical code: KIN/CHME/04/2020).
Results
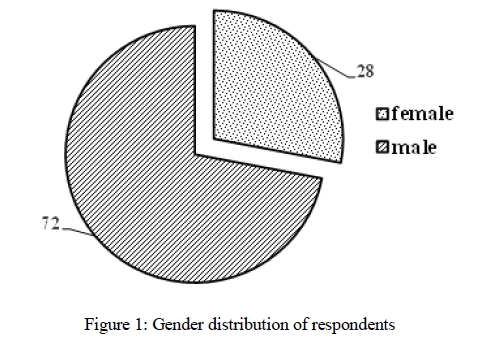
Most respondents are male (72%), and 28% are female. The distribution of respondents according to age group is described below. Figure 1 shows the distribution of respondents by gender.
It can be observed that 57.7% of respondents are in the 51-75 age group and 6.2% in the 0-25 age group. The relationship between normal and pathological values of different biological parameters is presented below.
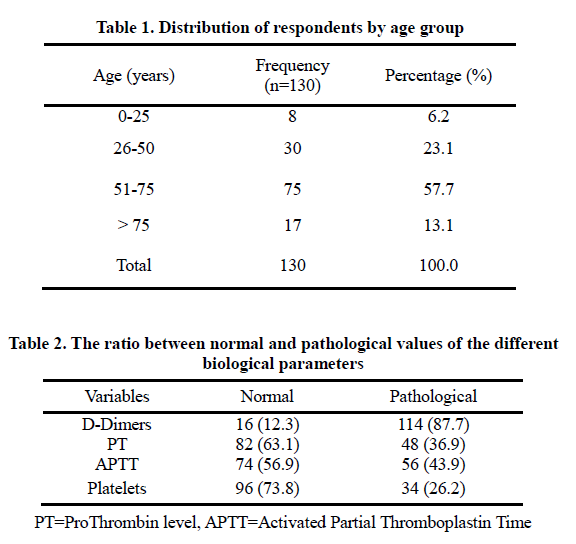
D-Dimers are more influential and/or pathological (87.7%) than the rest of the analyzed parameters.
The correlation between different biological parameters and socio-demographic characteristics is described below in Table 3.
It was observed that the 51-75 age group had a pathological D-dimer level (51.5%), a regular PT (40.8%), a normal APTT (36.2%), and a normal platelet count (41.5%). Among male respondents, the majority had a pathological D-dimer level (61.5%), while the remaining parameters were normal.

The symmetrical measurements between socio-demographic characteristics and D-dimers are presented in Table 4.
As the preliminary results are based on fewer than 1000 samples, it was observed that there is a positive relationship between age groups and D-dimer levels (n=130, r=0.233, and p=0.05). Similarly, a positive relationship exists between gender and D-dimer levels (n=130, r=0.081, and p=0.05).

Discussion
Regarding socio-demographic characteristics, it was observed that 72% of respondents were male. These findings are similar to those of Lumbulumbu (20), who reported the predominance of male respondents in Nord Kivu, which was also affected by COVID-19. In addition, it was observed that 57.7% of respondents were in the 51-75 age group, with a low representation of young people in the 0-25 age group (6.2%). This can be explained by the fact that more than 90% of deaths related to COVID-19 occur in the elderly due to various chronic pathologies and other medical histories. It is also due to the decrease in immune system efficiency when facing new pathogens that emerge or re-emerge compared to the youth with a very efficient immune system (21).
Different biomarkers of hemostasis were assessed in association with socio-demographic characteristics. The findings showed that the D-dimer assay presented very high values (>500 μg/L) in the 51-75 age group (51.6%) and among male respondents (61.5%). This study corroborates the findings of El Kettani (8), Tang et al. (10), Wu et al. (22), and Tang et al. (23).
Moreover, Tang et al. (23) reported that elevation of D-dimer levels (>500 μg/L) is frequent in this disease. The association between D-dimer elevation and disease severity has been confirmed, with a higher risk of mechanical ventilation or death (24). Tang et al. (10) noted that in deceased patients, there was a significant elevation of D-dimer and fibrin degradation products by 3.5 and 1.9 times, respectively, with a significant decrease in PT of 14%. Regarding the platelet count of respondents, it is usually normal or low (thrombocytopenia) on admission but may show dynamic changes during hospitalization. A low platelet count was identified as a poor prognostic factor in the elderly (25, 26). In this study, thrombocytopenia was noted in 26.2% of respondents. These findings are similar to those of Lamouasni (27), who also noted a low platelet count in patients affected by COVID-19. Thrombocytopenia is often considered an indicator of severity in sepsis. This also seems to be the case with SARS-CoV-2 infection, as thrombocytopenia was associated with a 5-fold higher risk of a severe form of the disease (28). SARS-CoV-2 inhibits hematopoiesis through CD13 receptors and can decrease the initial platelet formation, reduce many blood cells, and lead to thrombocytopenia (29).
It should be noted that PT and APTT are among the hemostasis parameters routinely measured and remain within normal values in most COVID-19 patients, including the most severe ones, both those hospitalized in intensive care and those presenting thrombotic events (30, 31). A decrease in PT may occur and suggest a diagnosis of disseminated intravascular coagulation (10). The APTT may indicate the presence of a lupus anticoagulant antibody (32), which is associated with a prothrombotic state.
In this study, the PT and APTT values were within the normal range for 63.1% and 56.9% of respondents, respectively. These findings are comparable to those of Helms et al. (33), who noted that 72% of PT and 67% of APTT were within normal limits in patients with COVID-19. Therefore, these standard coagulation parameters do not appear sufficient to assess COVID-19-induced coagulopathy; thus, exploring other blood parameters is necessary.
Conclusion
SARS-CoV-2 infection significantly impacts coagulation, with blood hypercoagulability being common in hospitalized individuals with COVID-19. These biological parameters could predict the risk of disease severity or death in people with COVID-19. These biomarkers will effectively help identify high-risk patients and allow for early and prolonged anticoagulation management. However, this study should be expanded by incorporating other important biomarkers such as Fibrinogen, Fibrin degradation products, etc., and utilizing other analytical techniques to enhance decision-making and ensure proper biological follow-up of COVID-19 patients.
Acknowledgement
Our sincere gratitude to the head of department of Medical Biology who allowed us to have access to patients’ files, to our colleagues in the service who gave a helpful hand while performing analysis.
Funding sources
Not applicable.
Ethical statement
An informed consent was sought from patients after explaining the purpose of the research. The confidentiality and anonymity of the patients' data were assured. The study was authorized by the Ethical Committee of CHME-Monkole (Ethical code: KIN/CHME/04/2020).
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Author contributions
MYM, GNB, NKN - Research concept and design, MYM, HN, EBB, YMM, JKM, CKK - Collection and/or assembly of data, MYM, JMV, GNB, NKN - Data analysis and interpretation, EBB, JNN, JIK -Writing the article, GNB, NKN –Critical revision of the article. All authors read and approved the final version of the article.
Coronavirus 2019 (COVID-19) is a viral infection caused by the severe acute respiratory syndrome virus (SARS-CoV-2) (1, 2). SARS-CoV-2 is a single-stranded RNA virus that enters the body's cells via the angiotensin-converting enzyme 2 (ACE2) receptor (3, 4). This receptor is widely expressed in the body, particularly in the pulmonary alveoli and vascular endothelium (5, 6). WHO statistics on the coronavirus pandemic have revealed over 317 million infected cases and over 5 million deaths worldwide. Like all countries, the Democratic Republic of the Congo (DRC) was also affected by the COVID-19 pandemic, with 81,719 infected cases and 1225 deaths (7).
The disease is transmitted through close contact with infected persons. Most infected individuals initially present with respiratory failure, but some progress to more systemic disease and multi-organ dysfunction. The elderly and those with co-morbidities are at an increased risk of death from COVID-19 (8). The clinical manifestations reported during COVID-19 infection are vast. Among them, an inflammatory state can be very significant and lead to a cytokine storm and a prothrombotic state, resulting in thrombosis (9). This inflammation causes damage to the activation of the microvascular endothelium, which is probably at the origin of the manifestations of pulmonary or renal pathologies (9). Notably, 5% to 30% of hospitalized patients develop a clinically proven thrombotic event. Emerging evidence suggests that endothelial damage resulting from cell invasion by SARS-CoV-2 and subsequent dysregulation of the host response involving the inflammation and coagulation pathways play a crucial role in the progression of severe COVID-19 (10,11).
The most severely ill patients frequently observe coagulopathy and massive intravascular clot formation of the disseminated intravascular coagulation type. Therefore, coagulation and fibrinolysis tests help identify and monitor severe cases of COVID-19. Increased D-dimer levels, a relatively modest decrease in platelet count, and prolonged prothrombin time are typical findings in patients with COVID-19 and coagulopathy (12). This hemostatic imbalance is thought to contribute to organ failure, multisystem involvement, and death (13). In France, in a series of 107 consecutive COVID-19 cases admitted to an intensive care unit, 20% developed venous thromboembolism, despite standard pharmacological thromboprophylaxis and full-dose anticoagulation therapy. COVID-19 patients with acute respiratory syndrome were 6 times more likely to develop pulmonary embolism (13). However, the optimal way to prevent and treat the coagulopathies that accompany COVID-19 would be to identify early markers of increased risk of thrombosis and the thrombotic complications that may ensue to help physicians prevent such complications (12, 13). The pathophysiology of microthrombosis is not yet well understood, and several hypotheses have been put forward. In the DRC, nearly 15% of COVID-19 patients are followed in different COVID-19 Treatment Centers. Of all these hospitalized patients, 41.4% were on oxygen in a severe and critical condition, all in Kinshasa, with a case fatality rate of 10% (14).
This study aimed to evaluate the coagulation disorders in COVID-19 patients admitted to Centre Hospitalier Mère et Enfant Monkole (CHME-Monkole), Kinshasa, DRC, from a biological perspective.
Methods
This descriptive cross-sectional hospital-based study on patient files was conducted between July 2020 and June 2021 at the Medical Biology Laboratory of CHME-Monkole in Mont-Ngafula municipality, Kinshasa, DRC.
As inclusion criteria, any person infected with the SARS-CoV-2 virus who was admitted to the hospital for therapeutic follow-up at CHME-Monkole and had samples sent to the Medical Biology Laboratory for analysis was included in the study. On the contrary, any person infected with SARS-CoV-2 who was not admitted and followed up at CHME was excluded from this study. The study population comprised patients with COVID-19 admitted to this health facility, and the sample size was 130 patients determined using a random sampling technique after conducting interviews based on a questionnaire.
For each respondent, epidemiological, clinical, biochemical, and hematological data and socio-demographic characteristics were collected using a questionnaire. The determination of Prothrombin time (PT), the Activated Partial Thromboplastin time (APTT), platelet count, and D-dimers were performed following the protocols of Mohammed et al. (15), Yorike et al. (16), Bashash et al. (17), Elkhalifa (18), and Long et al. (19) with slight modifications. The normal ranges for PT were 10-15 seconds converted to a percentage (70-100%), for APTT were 30-40 seconds, for platelet count was 150,000-400,000/μL of blood, and for D-dimer was less than 500 μg/L (FEU). These values were used to confirm abnormal cases and to find correlations between these biological parameters (PT, APTT, D-dimers, and platelet count) and socio-demographic characteristics.
Data entry was performed in Microsoft Excel and then exported to SPSS 25.0 for coding and analysis. A descriptive analysis (frequency and percentage) was conducted for socio-demographic characteristics, while Pearson correlation (Chi-square) was used to determine associations between socio-demographic characteristics and different biological parameters. Conversely, the correlation was applied to determine the relationship between socio-demographic characteristics and D-dimer levels. The significance level for statistical tests was p < 0.05, and the confidence interval was 95%.
For data collection in this study, informed consent was sought from patients after explaining the purpose of the research. The confidentiality and anonymity of the patients' data were assured. The study was authorized by the Ethical Committee of CHME-Monkole (Ethical code: KIN/CHME/04/2020).
Results
Most respondents are male (72%), and 28% are female. The distribution of respondents according to age group is described below. Figure 1 shows the distribution of respondents by gender.

It can be observed that 57.7% of respondents are in the 51-75 age group and 6.2% in the 0-25 age group. The relationship between normal and pathological values of different biological parameters is presented below.

D-Dimers are more influential and/or pathological (87.7%) than the rest of the analyzed parameters.
The correlation between different biological parameters and socio-demographic characteristics is described below in Table 3.
It was observed that the 51-75 age group had a pathological D-dimer level (51.5%), a regular PT (40.8%), a normal APTT (36.2%), and a normal platelet count (41.5%). Among male respondents, the majority had a pathological D-dimer level (61.5%), while the remaining parameters were normal.

The symmetrical measurements between socio-demographic characteristics and D-dimers are presented in Table 4.
As the preliminary results are based on fewer than 1000 samples, it was observed that there is a positive relationship between age groups and D-dimer levels (n=130, r=0.233, and p=0.05). Similarly, a positive relationship exists between gender and D-dimer levels (n=130, r=0.081, and p=0.05).

Discussion
Regarding socio-demographic characteristics, it was observed that 72% of respondents were male. These findings are similar to those of Lumbulumbu (20), who reported the predominance of male respondents in Nord Kivu, which was also affected by COVID-19. In addition, it was observed that 57.7% of respondents were in the 51-75 age group, with a low representation of young people in the 0-25 age group (6.2%). This can be explained by the fact that more than 90% of deaths related to COVID-19 occur in the elderly due to various chronic pathologies and other medical histories. It is also due to the decrease in immune system efficiency when facing new pathogens that emerge or re-emerge compared to the youth with a very efficient immune system (21).
Different biomarkers of hemostasis were assessed in association with socio-demographic characteristics. The findings showed that the D-dimer assay presented very high values (>500 μg/L) in the 51-75 age group (51.6%) and among male respondents (61.5%). This study corroborates the findings of El Kettani (8), Tang et al. (10), Wu et al. (22), and Tang et al. (23).
Moreover, Tang et al. (23) reported that elevation of D-dimer levels (>500 μg/L) is frequent in this disease. The association between D-dimer elevation and disease severity has been confirmed, with a higher risk of mechanical ventilation or death (24). Tang et al. (10) noted that in deceased patients, there was a significant elevation of D-dimer and fibrin degradation products by 3.5 and 1.9 times, respectively, with a significant decrease in PT of 14%. Regarding the platelet count of respondents, it is usually normal or low (thrombocytopenia) on admission but may show dynamic changes during hospitalization. A low platelet count was identified as a poor prognostic factor in the elderly (25, 26). In this study, thrombocytopenia was noted in 26.2% of respondents. These findings are similar to those of Lamouasni (27), who also noted a low platelet count in patients affected by COVID-19. Thrombocytopenia is often considered an indicator of severity in sepsis. This also seems to be the case with SARS-CoV-2 infection, as thrombocytopenia was associated with a 5-fold higher risk of a severe form of the disease (28). SARS-CoV-2 inhibits hematopoiesis through CD13 receptors and can decrease the initial platelet formation, reduce many blood cells, and lead to thrombocytopenia (29).
It should be noted that PT and APTT are among the hemostasis parameters routinely measured and remain within normal values in most COVID-19 patients, including the most severe ones, both those hospitalized in intensive care and those presenting thrombotic events (30, 31). A decrease in PT may occur and suggest a diagnosis of disseminated intravascular coagulation (10). The APTT may indicate the presence of a lupus anticoagulant antibody (32), which is associated with a prothrombotic state.
In this study, the PT and APTT values were within the normal range for 63.1% and 56.9% of respondents, respectively. These findings are comparable to those of Helms et al. (33), who noted that 72% of PT and 67% of APTT were within normal limits in patients with COVID-19. Therefore, these standard coagulation parameters do not appear sufficient to assess COVID-19-induced coagulopathy; thus, exploring other blood parameters is necessary.
Conclusion
SARS-CoV-2 infection significantly impacts coagulation, with blood hypercoagulability being common in hospitalized individuals with COVID-19. These biological parameters could predict the risk of disease severity or death in people with COVID-19. These biomarkers will effectively help identify high-risk patients and allow for early and prolonged anticoagulation management. However, this study should be expanded by incorporating other important biomarkers such as Fibrinogen, Fibrin degradation products, etc., and utilizing other analytical techniques to enhance decision-making and ensure proper biological follow-up of COVID-19 patients.
Acknowledgement
Our sincere gratitude to the head of department of Medical Biology who allowed us to have access to patients’ files, to our colleagues in the service who gave a helpful hand while performing analysis.
Funding sources
Not applicable.
Ethical statement
An informed consent was sought from patients after explaining the purpose of the research. The confidentiality and anonymity of the patients' data were assured. The study was authorized by the Ethical Committee of CHME-Monkole (Ethical code: KIN/CHME/04/2020).
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Author contributions
MYM, GNB, NKN - Research concept and design, MYM, HN, EBB, YMM, JKM, CKK - Collection and/or assembly of data, MYM, JMV, GNB, NKN - Data analysis and interpretation, EBB, JNN, JIK -Writing the article, GNB, NKN –Critical revision of the article. All authors read and approved the final version of the article.
Research Article: Research Article |
Subject:
Laboratory Sciences
Received: 2023/01/15 | Accepted: 2023/04/8 | Published: 2023/10/2 | ePublished: 2023/10/2
Received: 2023/01/15 | Accepted: 2023/04/8 | Published: 2023/10/2 | ePublished: 2023/10/2
References
1. Mpiana PT, Ngbolua KT, Tshibangu DST, Kilembe JT, Gbolo BZ, Mwanangombo DT, et al. Identification of potential inhibitors of SARS-CoV-2 main protease from Aloe vera compounds: A molecular docking study. Chem Phys Lett. 2020;754:137751. [View at Publisher] [DOI] [PMID] [Google Scholar]
2. Matondo A, Kilembe JT, Mwanangombo DT, Nsimba BM, Gbolo BZ, Bongo GN, et al. Facing COVID-19 via anti-inflammatory mechanism of action: Molecular docking and Pharmacokinetic studies of six anti-inflammatory compounds derived from Passiflora edulis. Journal of Computational Chemistry & Molecular Modeling. 2021;5(1):550-60. [View at Publisher] [DOI] [Google Scholar]
3. Mpiana PT, Ngbolua KN, Tshibangu DST, Kilembe JT, Gbolo BZ, Mwanangombo DT, et al. Aloe vera (L.) Burm. F. as a potential anti-COVID-19 plant: a mini-review of its antiviral activity. European Journal of Medicinal Plants. 2020;31(8):86-93. [View at Publisher] [DOI] [Google Scholar]
4. Ngbolua KN, Mbadiko CM, Matondo A, Bongo GN, Inkoto CL, Gbolo BZ, et al. Review on Ethno-botany, Virucidal Activity, Phytochemistry and Toxicology of Solanum genus: Potential Bio-resources for the Therapeutic Management of Covid-19. European Journal of Nutrition & Food Safety. 2020;12(7):35-48. [View at Publisher] [DOI] [Google Scholar]
5. Walls AC, Park YJ, Tortorici MA, Wall A, Guire AT, Vaesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;183(6):1735. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiostensin-conerting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive care Med. 2020;46(4):586-90. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Faucher M, Chevrier A, Gagnon C, Béland A, Corbeil JP. Suivre la propagation de Covid-19 à travers le monde, mise à jour en temps réel. Le Devoir. 2022;0(0):347-361. [View at Publisher] [Google Scholar]
8. El Kettani Z. Une méta-analyse : l'intérêt du dosage des D-Dimères dans la surveillance des patients atteints du Covid-19. Thèse de Master de Biotechnologie Médicale, Faculté de Médecine et de Pharmacie de Rabat, Université Mohammed V de Rabat. 2020;58pp. [Google Scholar]
9. Susen S, Tacquard CA, Godon A, Mansour A, Garrigue D, Nguyen P, et al. Traitement anticoagulant pour la prévention du risque thrombotique chez un patient hospitalisé avec Covid-19 et surveillance de l'hémostase. Propositions du GIHP et du GFHT. 2020:13-20. [View at Publisher] [Google Scholar]
10. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasie. 2020;18(4):844-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endothelitis in Covid-19. The Lancet. 2020;395(10234):1417-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Iba T, Levy JH, Levi M, Thacil J. Coagulopathy in Covid-19. Journal of Thrombosis and Haemostasis. 2020;18(9):2103-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Godoy LC, Goligher EC, Lawler PR, Slutsky AS, Zarychanski R. Prévoir et gérer la coagulopathie et les manifestations thrombotiques de la Covid-19 sévères. Canadian Medical Association Journal. 2020 ;192(50):E1816-22. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. WHO. Regional office for Africa. Epidémie de la maladie à coronavirus 2019 (Covid-19) en République Démocratique du Congo. Rapport de situation hébdomadaire. 2021. [View at Publisher] [Google Scholar]
15. Mohammed OIA, Abdalla MHA. Determination of Prothrombin Time, Activated Partial Thromboplastin Time and D-Dimer Levels among Malaria Infected Patients in Sinnar State, Sudan. Open Access Library Journal. 2019;6(12):e5975. [View at Publisher] [DOI] [Google Scholar]
16. Yorike D, Kurniawan MR, Syafaat M. Analysis of D-Dimer Level and Prothombin Time (PT) Activated Prothombin Thromboplastin (APTT) on Heparin Administration to COVID-19 Patients. Indonesian Journal of Medical Laboratory Science and Technology. 2022;4(1):91-8. [View at Publisher] [DOI] [Google Scholar]
17. Bashash D, Abolghasemi H, Salari S, Olfatifar M, Eshghi P, Akbari ME. Elevation of D-Dimer, But Not PT and aPTT, Reflects the Progression of COVID-19 Toward an Unfavorable Outcome: A Meta-Analysis. Iranian Journal of Blood & Cancer. 2020;12(2):47-53. [View at Publisher] [Google Scholar]
18. Elkhalifa AME. D-dimer as a predictive and prognostic marker among COVID-19 patients. Saudi Med J. 2022;43(7):723-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Long H, Nie L, Xiang X, Li H, Zhang X, Fu X, Ren H, Liu W, Wang Q and Wu Q. D-Dimer and Prothrombin Time Are the Significant Indicators of Severe COVID-19 and Poor Prognosis. BioMed Res Int. 2020;2020:6159720. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Lumbulumbu KF. Care analyse rapide de genre -Covid-19 : DRC-Nord-Kivu, Sud-Kivu et Kinshasa. Care DRC. 2020 :1-18. [View at Publisher]
21. Tristan V. Plus de 90 % des décès du Covid-19 surviennent chez les plus de 65 ans. Journal le FIGARO. 2021. [View at Publisher]
22. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-42. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. [View at Publisher] [DOI] [PMID] [Google Scholar]
24. Lippi G, Favaloro EJ. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120(5):876-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Kazemi E, Nejat RS, Ashkan F, Sheibani H. The laboratory findings and different Covid-19 severities: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2021;20(1):17. [View at Publisher] [DOI] [PMID] [Google Scholar]
26. Jiang SQ, Huang QF, Xie WM, Lv C, Quan XQ. The association between severe Covid-19 and low platelet count: evidence from 31 observational studies involving 7613 participants. Br J Haematol. 2020;190(1):e29-33. [View at Publisher] [DOI] [Google Scholar]
27. Lamouasni K. Biomarqueurs prédictifs de la sévérité de la Covid-19. Thèse de doctorat, Faculté de Médecine et de Pharmacie - Marrakech. Université CADI AYYAD. 2021 : 86 p. [Google Scholar]
28. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (Covid-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
29. Teimury A, Khameneh MT, Khaledi EM. Major coagulation disorders and parameters in COVID-19 patients. Eur J Med Res. 2022;27(1):25. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-20. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Ranucci M, Ballotta A, Dedda UD, Baryshnikova E, Poli MD, Resta M, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(7):1747-51. [View at Publisher] [DOI] [PMID] [Google Scholar]
32. Bowles L, Platon S, Yartey N, Dave M, Lee K, Hart DP, MacDonald V, Vert L, Sivapalaratnam S, Pasi KJ and Callum PC. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med. 2020;383(3):288-90. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. Helms J, Tacquard C, Sévérac F, Léonard-Lorant IL, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089-98. [View at Publisher] [DOI] [PMID] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.





